Theme Leads: Dr Pat Kiely, Dr Kieran McGourty
Overcoming the major challenges in cancer therapy will also require a better understanding of the complex interplay between the cancer cell and its microenvironment (TME), the rich diversity of different non-cancerous cell types and extracellular matrix components that comprise the tumour mass. A comprehensive understanding of these interactions has not been possible to date because previous studies have been unable to fully capture the complexity of tumours as evolving spatially related ecosystems. At UL we have an ambitious plan to tackle these shortcomings by applying a multi-disciplinary approach to the analysis of clinical samples obtained from longitudinal investigations of well-defined patient cohorts and combining this with the design and study of new more physiologically relevant models of the disease. This will more faithfully recapitulate the evolution of human cancers. Using the interdependent expertise of oncologists, pathologists, computational scientists, electronic and computer engineers and mathematicians, we will integrate the analysis of the complex datasets that emerge from these investigations to discover new biology that can drive a better understanding of disease and in turn drive the development of novel molecular diagnostic tests and targeted therapeutics for cancer patients. We will leverage UL‘s strengths in microfluidics, biomedical engineering and biomaterials science to develop new ‘tumour-on-chip’ models that will further enhance our understanding of cancer cell-TME interactions and improve our capabilities in drug development and drug delivery.
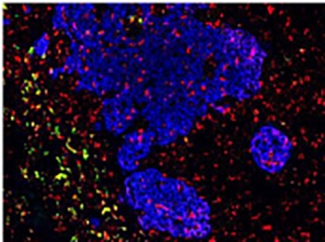
A) We are using single cell analysis and novel data interrogation and interpretation procedures to better understand the composition of the tumour. In parallel, we are designing interrogative methodologies that preserve the spatial information and the biological features of the tumour microenvironment. Applying genomic, proteomic and metabolic profiling to the spatially protected tumour will help us understand better how cancer cells behave and allow us to comment on tumour heterogeneity, the drug response and will help us design novel immune therapies and therapeutic approaches.
B) We are designing novel cell culture methods, protocols and platforms, to recapitulate the complexity of the tumour microenvironment. These methods such as ‘multicellular tumour spheroids’ and ‘tumour chips’ will allow is to alter parameters such as; cell composition, substrates such as collagen and fibronectin, and alter the biophysical parameters and properties of the culture environment. We are integrating cell monitoring approaches and bio sensors into our culture models to examine treatment dose and drug response. We are designing these methods to integrate with our extensive omics approaches to generate pipelines of data that will influence targeted therapy and precision oncology.
The role of the tumour microenvironment in cancer progression is one of the most exciting areas of cancer research. Unlocking the secrets of the tumour microenvironment will reveal information about the hierarchical clustering of cells within a tumour and help in the classification of tumours. Spatial multi-omics profiles and data driven cancer cell profiling will bring us closer to patient specific therapy
Understanding how the tumour microenvironment influences cancer cell progression, and designing approaches and methods that unlocking the secrets of the tumour microenvironment will help us understand and overcome tumour resistance to therapy, help design and enhance immunotherapies, help us enhance drug delivery and will reveal novel therapeutic strategies and approaches.
A. F. Mahdi, B. Malacrida, J. Nolan, M. E. McCumiskey, A. B. Merrigan, A. Lal, S. Tormey, A. J. Lowery, K. McGourty, P. A. Kiely, Expression of Annexin A2 Promotes Cancer Progression in Estrogen Receptor Negative Breast Cancers. Cells 9, (2020)
J. Nolan, A. F. Mahdi, C. P. Dunne, P. A. Kiely, Collagen and fibronectin promote an aggressive cancer phenotype in breast cancer cells but drive autonomous gene expression patterns. Gene 761, 145024 (2020)
S. Connolly, K. McGourty, D. Newport, The in vitro inertial positions and viability of cells in suspension under different in vivo flow conditions. Scientific reports 10, 1711 (2020)
S. Connolly, D. Newport, K. McGourty, The mechanical responses of advecting cells in confined flow. Biomicrofluidics 14, 031501 (2020)
M. Franzoni, D. T. O'Connor, L. Marcar, D. Power, M. A. Moloney, E. G. Kavanagh, R. L. Leask, J. Nolan, P. A. Kiely, M. T. Walsh, The Presence of a High Peak Feature Within Low-Average Shear Stimuli Induces Quiescence in Venous Endothelial Cells. Annals of biomedical engineering 48, 582-594 (2020)
J. Nolan, S. S. Dunne, W. Mustafa, L. Sivananthan, P. A. Kiely, C. P. Dunne, Proposed hypothesis and rationale for association between mastitis and breast cancer. Medical hypotheses 144, 110057 (2020)
R. K. Flygaard, B. Malacrida, P. Kiely, L. B. Jenner, Purification and characterization of native human elongation factor 2. Protein expression and purification 158, 15-19 (2019)
S. L. Lai, M. L. Tan, R. J. Hollows, M. Robinson, M. Ibrahim, S. Margielewska, E. K. Parkinson, A. Ramanathan, R. B. Zain, H. Mehanna, R. J. Spruce, W. Wei, I. Chung, P. G. Murray, L. F. Yap, I. C. Paterson, Collagen Induces a More Proliferative, Migratory and Chemoresistant Phenotype in Head and Neck Cancer via DDR1. Cancers 11, (2019)
D. R. Walsh, A. M. Ross, S. Malijauskaite, B. D. Flanagan, D. T. Newport, K. D. McGourty, J. J. E. Mulvihill, Regional mechanical and biochemical properties of the porcine cortical meninges. Acta biomaterialia 80, 237-246 (2018)
C. M. Dowling, S. L. Hayes, J. J. Phelan, M. C. Cathcart, S. P. Finn, B. Mehigan, P. McCormick, J. C. Coffey, J. O'Sullivan, P. A. Kiely, Expression of protein kinase C gamma promotes cell migration in colon cancer. Oncotarget 8, 72096-72107 (2017)
S. Hayes, B. Malacrida, M. Kiely, P. A. Kiely, Studying protein-protein interactions: progress, pitfalls and solutions. Biochemical Society transactions 44, 994-1004 (2016)
C. M. Dowling, J. Phelan, J. A. Callender, M. C. Cathcart, B. Mehigan, P. McCormick, T. Dalton, J. C. Coffey, A. C. Newton, J. O'Sullivan, P. A. Kiely, Protein kinase C beta II suppresses colorectal cancer by regulating IGF-1 mediated cell survival. Oncotarget 7, 20919-20933 (2016)
C. M. Dowling, P. A. Kiely, Targeting Protein Kinase C Downstream of Growth Factor and Adhesion Signalling. Cancers 7, 1271-1291 (2015)
C. M. Dowling, C. Herranz Ors, P. A. Kiely, Using real-time impedance-based assays to monitor the effects of fibroblast-derived media on the adhesion, proliferation, migration and invasion of colon cancer cells. Bioscience reports 34, (2014)